what type of covalent bond is co2 G) covalent substances – igcse chemistry revision
Carbon dioxide, or CO2, is a fascinating molecule that has widespread implications for everyday life. Understanding the atomic structure and chemical properties of CO2 is fundamental for scientists and ordinary people alike. First, let’s take a closer look at the atomic structure of carbon dioxide. As the name suggests, CO2 is composed of two oxygen atoms and one carbon atom. The bond between these atoms is a covalent bond, which means that electrons are shared between them. The shape of the molecule is linear, with the two oxygen atoms on either side of the central carbon atom. CO2 has several unique properties that make it essential for life on Earth. One of the most important is its role in the carbon cycle. During photosynthesis, plants take in CO2 from the atmosphere and use it to produce energy and oxygen. This oxygen is then released back into the atmosphere, while the carbon is used to build plant tissues. When plants die or are eaten, the carbon is returned to the atmosphere as CO2. This cycle is essential for maintaining the balance of gases in the atmosphere. CO2 is also a greenhouse gas, which means that it has the ability to trap heat in the atmosphere. This is a natural process that helps to regulate the temperature of the Earth. However, in recent years, human activities have increased the amount of CO2 in the atmosphere, leading to global warming and climate change. It is essential that we take steps to reduce our carbon footprint and reduce the amount of CO2 that is released into the atmosphere. Now, let’s take a closer look at the chemical properties of CO2. CO2 is a stable molecule that is relatively unreactive. This makes it safe for use in a wide range of applications, from carbonating drinks to fire extinguishers. CO2 is also used in many industrial processes, such as in the production of dry ice and for welding and cutting metals. Overall, carbon dioxide is a fascinating molecule that has widespread implications for our planet and everyday life. By understanding its atomic structure and chemical properties, we can better appreciate its importance and take steps to mitigate its impact on the environment. So the next time you crack open a cold soda or take a deep breath of fresh air, remember the important role that CO2 plays in our lives.
If you are searching about Reading: Covalent Bonds | Biology (Early Release) you’ve visit to the right page. We have 5 Pics about Reading: Covalent Bonds | Biology (Early Release) like Atomic Structure Of Carbon Dioxide - diariosdemusicman, g) Covalent Substances – IGCSE Chemistry revision and also Is CO2 Ionic or Covalent? - Techiescientist. Read more:
Reading: Covalent Bonds | Biology (Early Release)
 courses.lumenlearning.comcovalent bonds nonpolar bond polar molecule shape molecules carbon atoms hydrogen molecular type figure between biology water dioxide two oxygen
courses.lumenlearning.comcovalent bonds nonpolar bond polar molecule shape molecules carbon atoms hydrogen molecular type figure between biology water dioxide two oxygen
Is Carbon Dioxide (CO2) Polar Or Nonpolar? » Science ABC
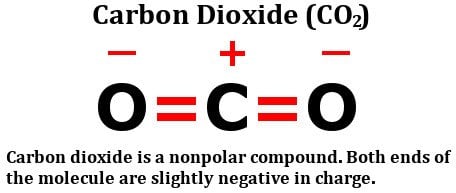 www.scienceabc.comcarbon dioxide co2 polar nonpolar structure bonds molecule bond look combined considered effect must let then these
www.scienceabc.comcarbon dioxide co2 polar nonpolar structure bonds molecule bond look combined considered effect must let then these
Atomic Structure Of Carbon Dioxide - Diariosdemusicman
diariosdemusicman.blogspot.comdioxide co2 exists
Is CO2 Ionic Or Covalent? - Techiescientist
 techiescientist.comcovalent co2 ionic techiescientist polarity bonds
techiescientist.comcovalent co2 ionic techiescientist polarity bonds
G) Covalent Substances – IGCSE Chemistry Revision
 igcsechemrevision.wordpress.comcarbon dioxide molecule covalent bond bonding chemical co2 structure electrons make sharing chemistry oxygen atom shell double ions shells chains
igcsechemrevision.wordpress.comcarbon dioxide molecule covalent bond bonding chemical co2 structure electrons make sharing chemistry oxygen atom shell double ions shells chains
Covalent co2 ionic techiescientist polarity bonds. Covalent bonds nonpolar bond polar molecule shape molecules carbon atoms hydrogen molecular type figure between biology water dioxide two oxygen. Is carbon dioxide (co2) polar or nonpolar? » science abc